Niobium has a bcc crystal structure – Niobium, a transition metal renowned for its exceptional properties, possesses a body-centered cubic (BCC) crystal structure, a defining characteristic that significantly influences its physical and mechanical behavior. This article delves into the intricacies of niobium’s BCC structure, exploring its implications for the metal’s properties and its diverse applications across various industries.
The BCC arrangement of atoms in niobium’s crystal lattice endows it with a unique combination of strength, ductility, and corrosion resistance. These properties make niobium an ideal material for applications demanding high performance and durability, such as in aerospace, energy, and medical sectors.
Crystal Structure

A body-centered cubic (BCC) crystal structure is a type of crystal structure in which the atoms are arranged in a cubic lattice with one atom at each corner and one atom in the center of the cube.
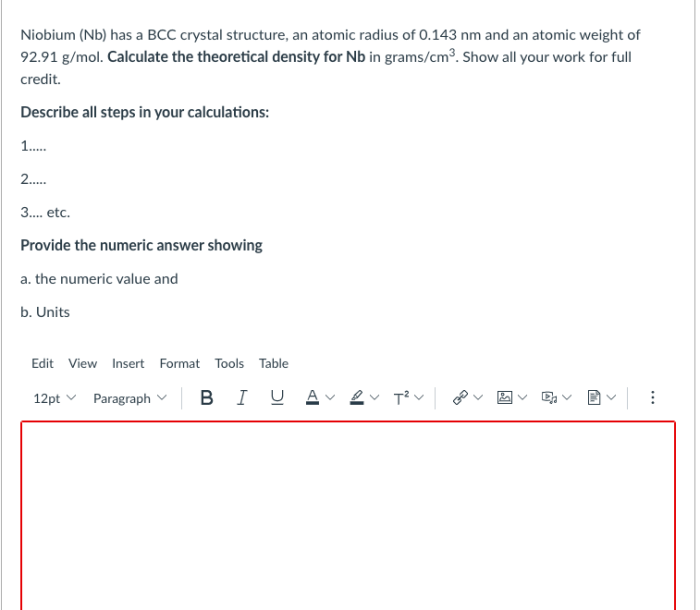
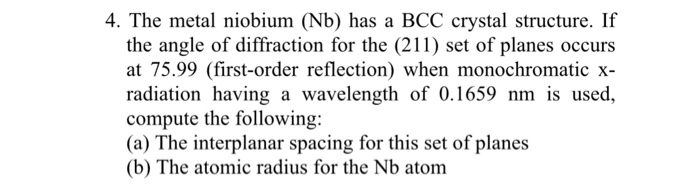
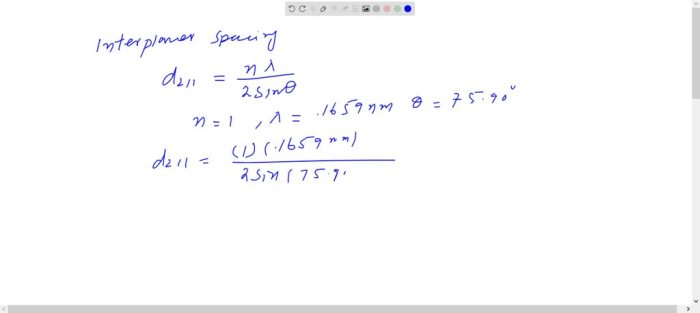
Arrangement of Atoms
In a BCC crystal structure, the atoms are arranged in a repeating pattern of cubes. Each cube is surrounded by eight other cubes, and each atom is surrounded by eight other atoms. The atoms are arranged in a way that maximizes the number of atoms that can be packed into a given space.
The following illustration shows a BCC crystal structure:
Niobium’s BCC Structure
Niobium crystallizes in a body-centered cubic (BCC) structure, where atoms are arranged in a cubic lattice with one atom at each corner and one atom in the center of the cube. This crystal structure has a coordination number of 8, meaning that each niobium atom is surrounded by eight nearest neighbors.
The BCC structure of niobium has a significant influence on its physical and mechanical properties. The close-packed arrangement of atoms in the BCC structure gives niobium high strength and stiffness, making it a strong and durable material. Additionally, the BCC structure allows for easy dislocation movement, resulting in good ductility and toughness.
Applications
Niobium’s BCC structure makes it suitable for various applications where strength, stiffness, and toughness are required. Some examples include:
- Aerospace:Niobium alloys are used in aircraft engines and spacecraft components due to their high strength-to-weight ratio and ability to withstand extreme temperatures.
- Medical:Niobium is used in surgical implants and medical devices due to its biocompatibility and corrosion resistance.
- Superconductivity:Niobium-titanium alloys are used in superconducting magnets, which are essential components in MRI scanners and particle accelerators.
Comparison to Other Structures

The BCC crystal structure of niobium is one of the most common crystal structures found in metals. It is characterized by a cubic unit cell with two atoms at each corner and one atom in the center. This structure is very similar to the FCC crystal structure, which is also found in many metals.
However, there are some key differences between the two structures.
One of the most significant differences between the BCC and FCC crystal structures is the number of nearest neighbors each atom has. In the BCC structure, each atom has eight nearest neighbors, while in the FCC structure, each atom has twelve nearest neighbors.
This difference in the number of nearest neighbors can have a significant impact on the properties of the material.
Stacking Sequence, Niobium has a bcc crystal structure
Another difference between the BCC and FCC crystal structures is the stacking sequence of the atomic layers. In the BCC structure, the atomic layers are stacked in an ABCABC pattern, while in the FCC structure, the atomic layers are stacked in an ABCAB pattern.
This difference in the stacking sequence can also affect the properties of the material.
Properties
The BCC crystal structure is generally stronger and harder than the FCC crystal structure. This is because the BCC structure has a higher density of dislocations, which are defects in the crystal lattice that can make the material weaker. However, the FCC structure is more ductile than the BCC structure.
This is because the FCC structure has a lower stacking fault energy, which makes it easier for the material to deform plastically.
Applications and Properties
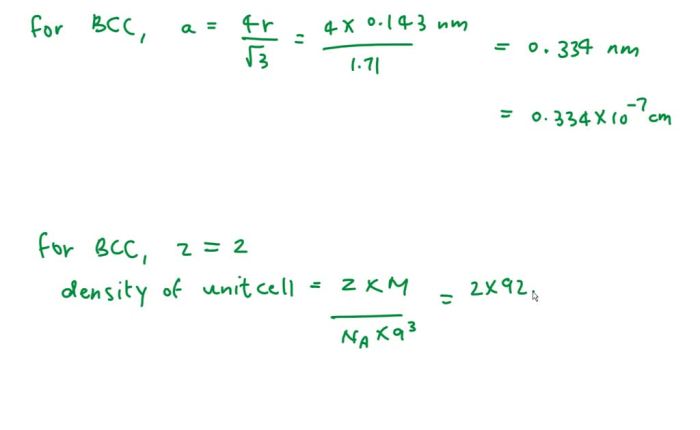
Niobium’s body-centered cubic (BCC) crystal structure plays a crucial role in its unique properties and wide range of applications. This structure imparts exceptional strength, ductility, and corrosion resistance to niobium.
Strength and Ductility
The BCC structure of niobium contributes to its exceptional strength and ductility. The regular arrangement of atoms in the BCC lattice provides strong interatomic bonds, making niobium resistant to deformation and fracture. This combination of strength and ductility makes niobium suitable for applications requiring both high load-bearing capacity and resistance to impact.
For instance, niobium alloys are used in the construction of high-performance aircraft components, such as jet engine blades and landing gear, where they withstand extreme mechanical stresses and temperatures.
Corrosion Resistance
The BCC structure of niobium also enhances its corrosion resistance. The tightly packed arrangement of atoms in the BCC lattice forms a protective oxide layer on the surface of the metal, preventing further oxidation and corrosion.
Niobium’s exceptional corrosion resistance makes it an ideal material for applications in harsh environments, such as chemical processing equipment, marine components, and biomedical implants. For example, niobium is used in the production of chemical pumps and valves, where it resists the corrosive effects of acids and alkalis.
Superconductivity
Although not directly related to its BCC structure, niobium is also known for its superconductivity properties. When cooled below a critical temperature, niobium exhibits zero electrical resistance, allowing for the efficient transmission of electricity without energy loss.
Niobium-based superconductors are used in a variety of applications, including high-energy accelerators, magnetic resonance imaging (MRI) machines, and superconducting power transmission cables.
Advanced Research: Niobium Has A Bcc Crystal Structure
Niobium’s unique BCC crystal structure continues to be a subject of active research, driven by its exceptional properties and potential applications in emerging technologies.
One area of ongoing research focuses on understanding the fundamental mechanisms governing the deformation and strengthening of niobium’s BCC structure. This involves studying the role of dislocations, grain boundaries, and other microstructural features in determining the material’s mechanical properties.
Advanced Applications
The unique properties of niobium’s BCC structure have led to its exploration for a variety of advanced applications. One promising area is in the development of high-performance superconducting materials. Niobium-based alloys are being investigated for use in superconducting magnets for fusion reactors, particle accelerators, and other energy-intensive applications.
Additionally, niobium’s BCC structure shows promise for use in lightweight and high-strength materials. Research is underway to develop niobium-based composites and alloys for applications in aerospace, automotive, and defense industries.
Future Directions
Future research on niobium’s BCC structure is expected to focus on exploring its potential in emerging technologies such as quantum computing, spintronics, and energy storage. The development of new characterization techniques and computational modeling tools will play a crucial role in advancing our understanding of this complex material.
Furthermore, research is expected to continue on the development of novel processing techniques to control the microstructure and properties of niobium-based materials. This will enable the tailoring of materials for specific applications and the realization of their full potential.
Answers to Common Questions
What is the significance of niobium’s BCC crystal structure?
Niobium’s BCC structure contributes to its exceptional strength, ductility, and corrosion resistance, making it suitable for demanding applications.
How does niobium’s BCC structure compare to other crystal structures?
BCC differs from FCC and HCP structures in terms of atomic arrangement and properties, influencing material characteristics such as strength and ductility.
What are some examples of applications where niobium’s BCC structure is advantageous?
Niobium’s BCC structure finds applications in aerospace components, superconducting magnets, and medical implants due to its strength and corrosion resistance.