Embark on a scientific expedition with the collision theory gizmo answer key, an indispensable tool that unlocks the secrets of chemical reactions. This guide delves into the fundamental principles, experimental setup, and practical applications of the Collision Theory, empowering you with a comprehensive understanding of reaction rates and their significance in various scientific disciplines.
Through a detailed exploration of the experimental setup, data collection techniques, and analysis methods, this guide provides a hands-on approach to testing and verifying the Collision Theory. By identifying the key factors that influence reaction rates, you will gain insights into the dynamics of chemical reactions and how they can be manipulated to optimize processes and enhance efficiency.
Collision Theory Gizmo Introduction
The Collision Theory Gizmo is a virtual simulation that allows students to explore the factors that affect the rate of chemical reactions. The gizmo is based on the Collision Theory, which states that in order for a chemical reaction to occur, the reacting molecules must collide with each other with sufficient energy to overcome the activation energy barrier.
The Collision Theory Gizmo allows students to control the temperature, concentration, and surface area of the reactants, and observe how these factors affect the rate of the reaction. The gizmo also allows students to track the number of collisions that occur between the reactants and the number of reactions that occur.
Experimental Setup
The experimental setup in the Collision Theory Gizmo is simple. The gizmo consists of a reaction chamber that contains the reactants. The temperature of the reaction chamber can be controlled using a thermostat. The concentration of the reactants can be controlled by adding or removing reactants from the reaction chamber.
The surface area of the reactants can be controlled by changing the size of the reaction chamber.
Data Collection and Analysis: Collision Theory Gizmo Answer Key
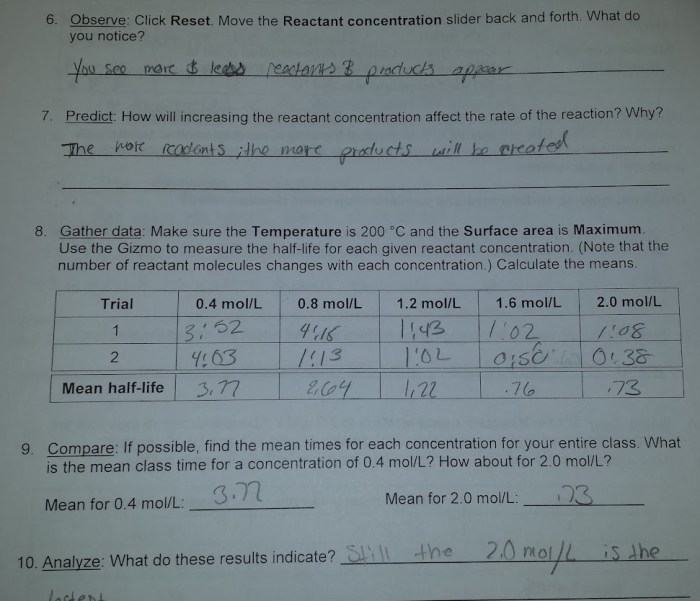
Data is collected in the Collision Theory Gizmo by tracking the number of collisions that occur between the reactants and the number of reactions that occur. The data is then plotted on a graph. The graph can be used to determine the rate of the reaction.
Factors Affecting Reaction Rates
The Collision Theory states that the rate of a chemical reaction is proportional to the number of collisions that occur between the reactants and the fraction of those collisions that have sufficient energy to overcome the activation energy barrier.
- Temperature:The rate of a reaction increases with increasing temperature. This is because the higher the temperature, the more energy the reactants have, and the more likely they are to collide with each other with sufficient energy to overcome the activation energy barrier.
- Concentration:The rate of a reaction increases with increasing concentration of the reactants. This is because the higher the concentration of the reactants, the more likely they are to collide with each other.
- Surface area:The rate of a reaction increases with increasing surface area of the reactants. This is because the larger the surface area of the reactants, the more likely they are to collide with each other.
Applications of Collision Theory
The Collision Theory has a wide range of applications in science and industry. The theory is used to design and optimize chemical reactors, to predict the rates of chemical reactions, and to understand the mechanisms of chemical reactions.
- Chemical reactors:The Collision Theory is used to design and optimize chemical reactors. By controlling the temperature, concentration, and surface area of the reactants, it is possible to maximize the rate of the reaction and minimize the production of unwanted byproducts.
- Predicting reaction rates:The Collision Theory can be used to predict the rates of chemical reactions. By knowing the temperature, concentration, and surface area of the reactants, it is possible to use the Collision Theory to calculate the rate of the reaction.
- Understanding reaction mechanisms:The Collision Theory can be used to understand the mechanisms of chemical reactions. By studying the effects of temperature, concentration, and surface area on the rate of a reaction, it is possible to determine the steps involved in the reaction and the rate-determining step.
Limitations of Collision Theory
The Collision Theory is a simple model that can be used to explain the rates of many chemical reactions. However, the theory has some limitations. The theory does not take into account the effects of solvent, the effects of catalysts, and the effects of reaction intermediates.
In addition, the theory does not apply to reactions that occur in the gas phase.
FAQ Summary
What is the Collision Theory?
The Collision Theory states that chemical reactions occur when particles collide with sufficient energy and proper orientation to form new products.
How does the Collision Theory Gizmo help in understanding reaction rates?
The Collision Theory Gizmo provides an interactive simulation that allows users to manipulate variables such as temperature, concentration, and particle size to observe their effects on reaction rates.
What are the key factors that affect reaction rates according to the Collision Theory?
According to the Collision Theory, the key factors that affect reaction rates include temperature, concentration, particle size, and the presence of a catalyst.