Delving into the fascinating realm of cell biology, the cell membrane and tonicity worksheet embarks on an enlightening journey, unraveling the intricate structure and functions of cell membranes and their pivotal role in maintaining cellular homeostasis. This comprehensive guide promises to illuminate the fundamental concepts of tonicity, water movement, and their profound impact on cell physiology.
The cell membrane, a delicate yet robust barrier, governs the passage of substances into and out of cells, safeguarding their internal environment. Tonicity, a measure of the solute concentration gradient across a semipermeable membrane, dictates the direction and magnitude of water movement, shaping cell volume and function.
Understanding these principles is paramount in comprehending a wide range of biological processes, from cellular respiration to disease pathogenesis.
Cell Membrane and Tonicity

The cell membrane, also known as the plasma membrane, is a selectively permeable barrier that surrounds all living cells. It regulates the passage of materials into and out of the cell, maintaining the cell’s internal environment and protecting it from its surroundings.
Understanding the structure and function of the cell membrane is crucial for comprehending cellular processes and their implications in various biological contexts.
Cell Membrane Structure
The cell membrane is composed primarily of a phospholipid bilayer, a double layer of phospholipids arranged tail-to-tail. Phospholipids are amphipathic molecules, meaning they have both hydrophilic (water-loving) and hydrophobic (water-hating) regions. The hydrophobic tails of the phospholipids face inward, away from the aqueous environment, while the hydrophilic heads face outward, interacting with water.Embedded
within the phospholipid bilayer are cholesterol molecules and proteins. Cholesterol helps to maintain the fluidity and stability of the membrane, while proteins perform various functions, including transport, signaling, and cell adhesion.
Tonicity and Water Movement
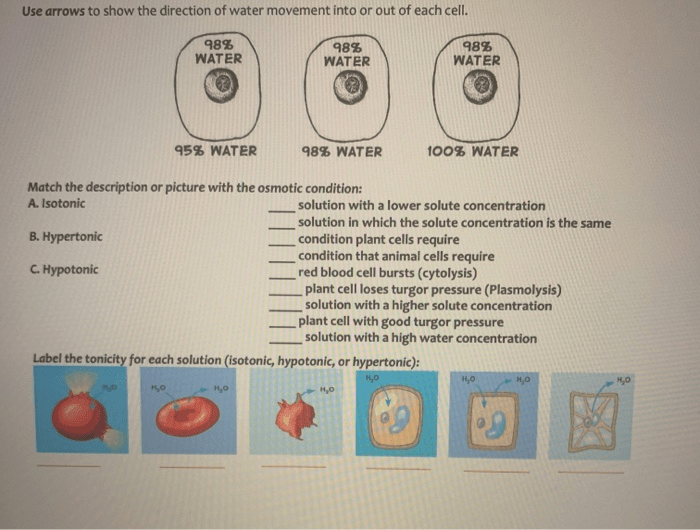
Tonicity refers to the relative concentration of solutes in a solution compared to another solution. When two solutions have the same concentration of solutes, they are said to be isotonic. If one solution has a higher concentration of solutes than the other, it is said to be hypertonic.
Conversely, if one solution has a lower concentration of solutes than the other, it is said to be hypotonic.The tonicity of a solution affects the movement of water across the cell membrane. Water moves from an area of low solute concentration (hypotonic solution) to an area of high solute concentration (hypertonic solution) in an attempt to equalize the concentrations.
This process is known as osmosis.
Effects of Tonicity on Cells
The tonicity of the surrounding environment can have significant effects on cells.Effects on Animal Cells:
- Hypertonic solutions cause animal cells to shrink as water moves out of the cell.
- Hypotonic solutions cause animal cells to swell and potentially burst as water moves into the cell.
Effects on Plant Cells:
- Hypertonic solutions cause plant cells to plasmolyze, where the cell membrane pulls away from the cell wall.
- Hypotonic solutions cause plant cells to become turgid, where the cell membrane pushes against the cell wall.
Cell Membrane Permeability
The cell membrane is selectively permeable, meaning it allows certain substances to cross more easily than others. Small, nonpolar molecules, such as oxygen and carbon dioxide, can diffuse directly across the phospholipid bilayer. Larger, polar molecules, such as glucose and ions, require the assistance of membrane proteins to cross.The
permeability of the cell membrane is affected by several factors, including:
- The size and polarity of the molecule
- The presence of membrane proteins
- The temperature
Active and Passive Transport
Active and passive transport are two distinct mechanisms by which substances can move across the cell membrane.Active Transport:
- Requires energy (usually in the form of ATP)
- Moves substances against their concentration gradient (from low to high concentration)
- Mediated by carrier proteins
Passive Transport:
- Does not require energy
- Moves substances down their concentration gradient (from high to low concentration)
- Can occur through diffusion, osmosis, or facilitated diffusion
Tonicity in Different Environments, Cell membrane and tonicity worksheet
The tonicity of different environments can vary greatly. For example:| Environment | Tonicity ||—|—|| Freshwater | Hypotonic || Saltwater | Hypertonic || Human body | Isotonic |Organisms have adapted to live in environments with different tonicities. For example, marine organisms have evolved mechanisms to prevent water loss in hypertonic environments, while freshwater organisms have evolved mechanisms to prevent water gain in hypotonic environments.
Clinical Applications
The understanding of cell membrane and tonicity has important applications in medicine. For example:
- Dehydration occurs when the body loses too much water, resulting in hypertonic conditions within cells.
- Edema occurs when there is an excess of water in the body, resulting in hypotonic conditions within cells.
- Tonicity is also important in the development of new drugs and therapies that target the cell membrane.
FAQ Corner: Cell Membrane And Tonicity Worksheet
What is the role of cholesterol in the cell membrane?
Cholesterol contributes to membrane fluidity and stability, preventing excessive rigidity or fluidity.
How does tonicity affect plant cells?
In hypotonic solutions, plant cells swell due to water influx, while in hypertonic solutions, they shrink as water moves out.
What is the difference between active and passive transport?
Active transport requires energy to move substances against their concentration gradient, while passive transport occurs down the concentration gradient without energy input.