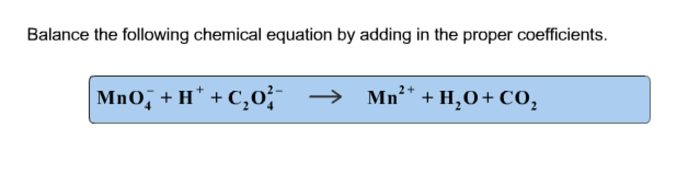
Balance the following chemical equations brainly – Balancing chemical equations is a fundamental skill in chemistry that allows us to understand and predict the behavior of chemical reactions. This guide provides a comprehensive overview of the topic, covering the definition and importance of balanced equations, different balancing methods, and their applications in various fields.
Whether you’re a student, researcher, or professional, this guide will equip you with the knowledge and techniques to confidently balance chemical equations and gain a deeper understanding of chemical reactions.
Chemical Equations
A chemical equation is a symbolic representation of a chemical reaction. It shows the reactants, the substances that undergo the reaction, and the products, the substances that are formed as a result of the reaction. Chemical equations are important because they allow us to predict the products of a reaction and to calculate the amounts of reactants and products that are involved.
Balancing Chemical Equations: Balance The Following Chemical Equations Brainly
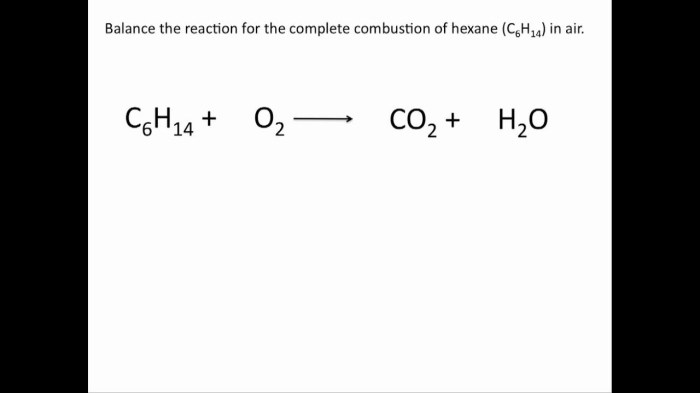
Chemical equations must be balanced in order to be accurate. A balanced chemical equation has the same number of atoms of each element on both sides of the equation. Balancing chemical equations can be done using a variety of methods, including the inspection method, the half-reaction method, and the oxidation number method.
Inspection Method, Balance the following chemical equations brainly
The inspection method is the simplest method for balancing chemical equations. It involves looking at the equation and adjusting the coefficients in front of each reactant and product until the equation is balanced. For example, the following equation is unbalanced:
“`
- H2 + O2
- > H2O
“`
To balance this equation, we can adjust the coefficients as follows:
“`
- H2 + O2
- > 2H2O
“`
This equation is now balanced because there are the same number of atoms of each element on both sides of the equation.
| Unbalanced Equation | Balanced Equation |
|---|---|
2H2 + O2
|
2H2 + O2
|
CH4 + 2O2
|
CH4 + 2O2
|
2Al + 3Cl2
|
2Al + 3Cl2
|
Applications of Balanced Chemical Equations
Balanced chemical equations have a wide range of applications, including:
- Stoichiometry: Balanced chemical equations can be used to calculate the amounts of reactants and products that are involved in a reaction.
- Reaction prediction: Balanced chemical equations can be used to predict the products of a reaction.
- Chemical synthesis: Balanced chemical equations can be used to design and optimize chemical synthesis processes.
Common Errors

There are a number of common errors that can be made when balancing chemical equations. These errors include:
- Not balancing the equation for all elements.
- Changing the subscripts of the reactants or products.
- Using fractional coefficients.
These errors can lead to incorrect predictions about the products of a reaction and the amounts of reactants and products that are involved.
Q&A
What is a balanced chemical equation?
A balanced chemical equation is an equation in which the number of atoms of each element on the reactants’ side is equal to the number of atoms of that element on the products’ side.
Why is it important to balance chemical equations?
Balancing chemical equations is important because it ensures that the law of conservation of mass is obeyed. This law states that mass can neither be created nor destroyed, so the total mass of the reactants must be equal to the total mass of the products.
What are the different methods for balancing chemical equations?
There are several methods for balancing chemical equations, including the inspection method, the half-reaction method, and the oxidation number method.