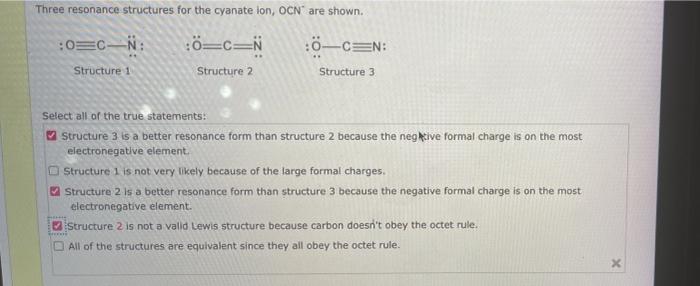
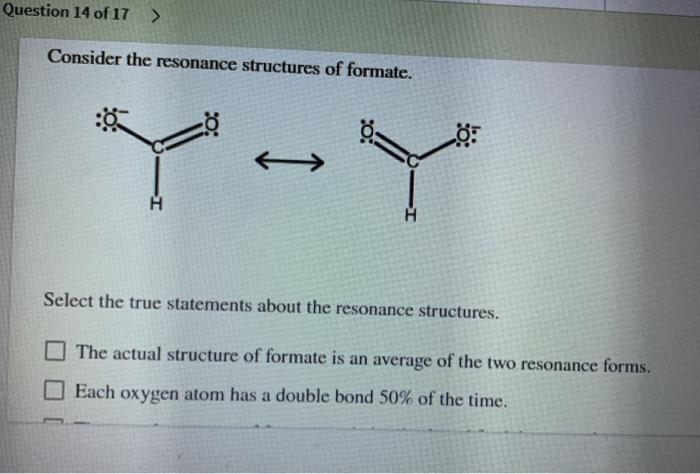
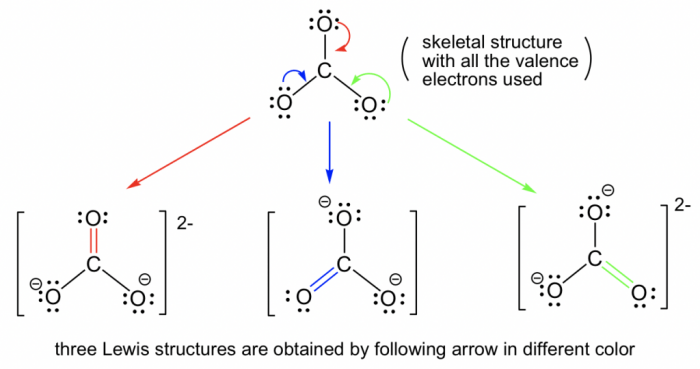
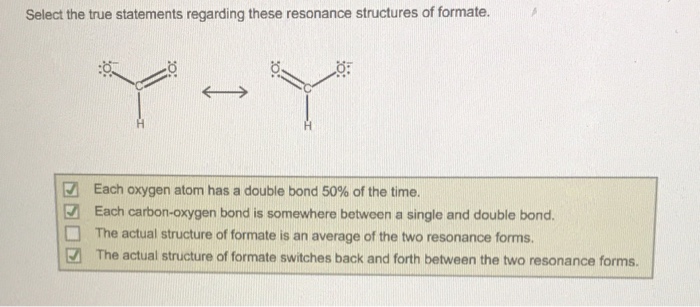
Select the true statements about the resonance structures. – Resonance structures, pivotal in chemistry, provide a profound understanding of molecular bonding and behavior. This article delves into the true statements surrounding resonance structures, exploring their significance in deciphering molecular stability, reactivity, and various chemical phenomena.
Resonance structures arise from the concept of delocalized electrons, where electrons are not confined to specific atoms but rather spread across multiple atoms. This delocalization contributes to the stability of molecules, as it lowers their overall energy and enhances their resonance energy.
Resonance Structures
Resonance structures are a way of representing the delocalization of electrons in a molecule. Delocalization occurs when electrons are not confined to a single atom or bond, but instead are spread out over a larger region of the molecule. This can happen when there are multiple equivalent Lewis structures for a molecule, or when there is a resonance hybrid that is a combination of two or more Lewis structures.
Explain Resonance Structures
Resonance structures are used to explain the bonding in molecules that cannot be adequately represented by a single Lewis structure. For example, the benzene molecule has six carbon atoms arranged in a ring, with one hydrogen atom attached to each carbon atom.
The Lewis structure of benzene shows each carbon atom bonded to its neighboring carbon atoms by a single bond and to the hydrogen atom by a single bond. However, this Lewis structure does not accurately represent the bonding in benzene, as it suggests that the carbon-carbon bonds are all equivalent.
In reality, the carbon-carbon bonds in benzene are all equivalent, and the electrons are delocalized over the entire ring.
Identify True Statements about Resonance Structures
- Resonance structures are a way of representing the delocalization of electrons in a molecule.
- Resonance structures can be used to explain the bonding in molecules that cannot be adequately represented by a single Lewis structure.
- Resonance structures are not real structures, but are instead a way of representing the average electron distribution in a molecule.
Compare Resonance Structures, Select the true statements about the resonance structures.
Resonance structures can be compared by their resonance energy. Resonance energy is the difference in energy between the resonance hybrid and the most stable Lewis structure. The greater the resonance energy, the more stable the resonance hybrid.
Applications of Resonance Structures
Resonance structures are used to explain a wide variety of chemical phenomena, including the stability of molecules, the reactivity of molecules, and the spectra of molecules.
Limitations of Resonance Structures
Resonance structures are not always able to accurately represent the bonding in a molecule. For example, resonance structures cannot be used to represent the bonding in molecules that have unpaired electrons.
Top FAQs: Select The True Statements About The Resonance Structures.
What is the significance of resonance structures?
Resonance structures provide a deeper understanding of molecular bonding and stability, enabling chemists to predict molecular properties and explain chemical reactions.
How can resonance structures be used to predict molecular properties?
Resonance structures can be used to estimate bond lengths, bond angles, and other molecular properties by considering the contributions of different resonance forms.
What are the limitations of resonance structures?
Resonance structures may not accurately represent all aspects of molecular bonding, particularly in cases where there is significant ionic character or when the molecule is highly polar.