Embark on a journey into the realm of chemical equations with our comprehensive Intro to Balancing Equations Worksheet Answer Key. This guide unveils the intricacies of chemical reactions, empowering you to master the art of balancing equations with precision and confidence.
Within these pages, you’ll delve into the fundamental concepts of chemical equations, unraveling their significance in representing chemical transformations. We’ll meticulously guide you through a step-by-step process for balancing equations, illuminating each step with illustrative examples.
Introduction to Balancing Equations: Intro To Balancing Equations Worksheet Answer Key
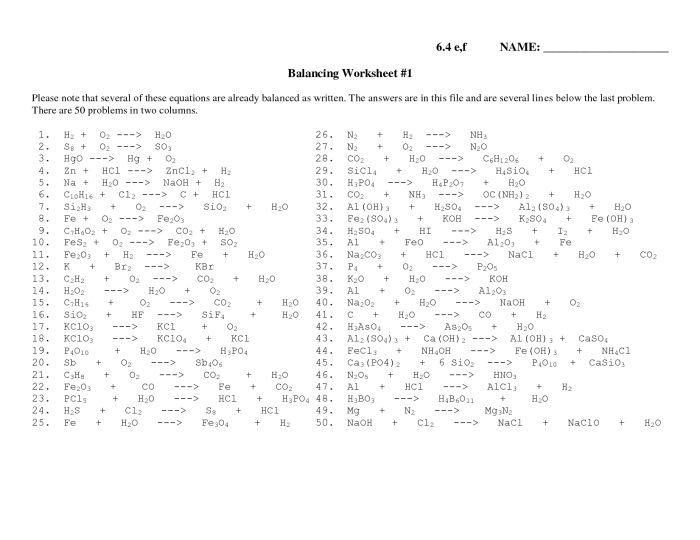
Chemical equations are symbolic representations of chemical reactions. They show the reactants, products, and the stoichiometric coefficients that balance the equation. Balancing chemical equations is essential to ensure that the number of atoms of each element is the same on both sides of the equation, which is required by the law of conservation of mass.
Steps for Balancing Equations, Intro to balancing equations worksheet answer key
Balancing chemical equations involves the following steps:
- Identify the unbalanced equation.
- Count the number of atoms of each element on both sides of the equation.
- Adjust the stoichiometric coefficients in front of each compound to balance the number of atoms of each element.
- Check if the equation is balanced by counting the atoms again.
Example:
Unbalanced equation: CH 4+ 2O 2→ CO 2+ H 2O
Balanced equation: CH 4+ 2O 2→ CO 2+ 2H 2O
Popular Questions
What is the significance of balancing chemical equations?
Balancing chemical equations ensures that the number of atoms of each element on the reactants’ side matches the number of atoms of the same element on the products’ side. This adherence to the law of conservation of mass is crucial for accurate chemical calculations and understanding reaction stoichiometry.
How do I approach balancing chemical equations?
Balancing chemical equations involves adjusting the stoichiometric coefficients in front of each chemical formula until the number of atoms of each element is identical on both sides of the equation. This iterative process requires careful observation and manipulation of coefficients to achieve a balanced state.