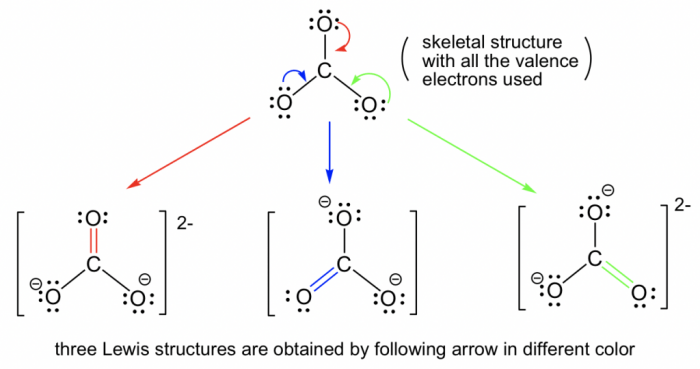
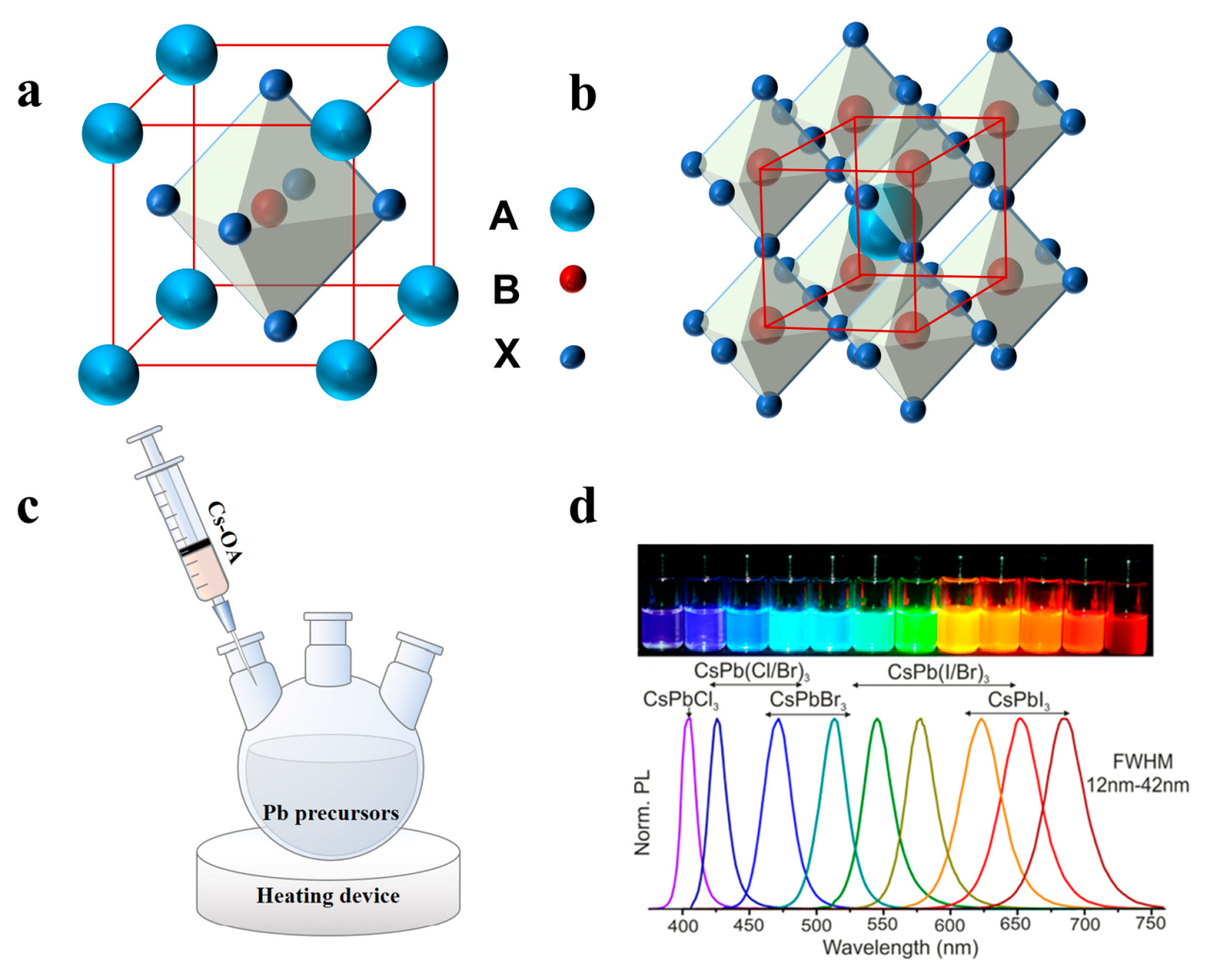
I am a halide with 4 energy levels – Halides with 4 energy levels, standing tall in the realm of chemistry, unveil a captivating tapestry of properties and applications. Their electronic structure, intricately interwoven with 4 distinct energy levels, sets them apart, granting them a unique identity in the world of halides.
Delve into this comprehensive exploration as we unravel the mysteries surrounding these remarkable compounds.
Their physical and chemical attributes, shaped by their exceptional electronic configuration, present a fascinating contrast to their halide counterparts with varying energy level configurations. Join us on this journey as we uncover the practical applications where halides with 4 energy levels shine, highlighting their specific advantages and limitations in these diverse fields.
Electronic Structure of Halides
Halides are a group of elements that belong to Group 17 of the periodic table. They have seven valence electrons, which means they are highly reactive and can easily form chemical bonds with other elements.The electronic structure of halides can be explained using the concept of energy levels.
Energy levels are discrete levels of energy that electrons can occupy within an atom. The energy levels are arranged in shells, with each shell having a specific number of subshells. The subshells are designated by the letters s, p, d, and f.Halides
with four energy levels have the following electronic configuration:“`
s22s 22p 63s 23p 5
“`The first energy level consists of the 1s subshell, which can hold up to two electrons. The second energy level consists of the 2s and 2p subshells, which can hold up to two and six electrons, respectively. The third energy level consists of the 3s and 3p subshells, which can hold up to two and six electrons, respectively.
The fourth energy level consists of the 4s subshell, which can hold up to two electrons.The valence electrons of halides are located in the outermost energy level, which is the fourth energy level in this case. The valence electrons are responsible for the chemical properties of halides.
Properties of Halides with 4 Energy Levels
Halides with 4 energy levels exhibit distinct physical and chemical properties that set them apart from halides with different energy level configurations. These properties are primarily influenced by the arrangement of electrons within their energy levels and the resulting electronic structure.
Physical Properties, I am a halide with 4 energy levels
- Color:Halides with 4 energy levels tend to be colorless or white in their pure form. However, they may exhibit different colors when combined with other elements or in certain compounds.
- Melting and Boiling Points:These halides generally have higher melting and boiling points compared to halides with fewer energy levels. This is due to the stronger intermolecular forces resulting from the increased number of electrons.
- Density:Halides with 4 energy levels are typically denser than halides with lower energy level configurations. The additional electrons contribute to a higher mass-to-volume ratio.
Chemical Properties
- Reactivity:Halides with 4 energy levels are generally less reactive than halides with fewer energy levels. The electrons in the higher energy levels are more stable and less likely to participate in chemical reactions.
- Oxidation States:These halides can exhibit multiple oxidation states, including -1, +1, +3, and +5. The ability to access different oxidation states allows them to participate in a wider range of chemical reactions.
- Complex Formation:Halides with 4 energy levels readily form complexes with transition metals. The electrons in the higher energy levels can donate to the metal ion, forming coordinate bonds.
Applications of Halides with 4 Energy Levels
Halides with 4 energy levels, particularly those from Group 7 (halogens), find diverse applications in various fields due to their unique electronic properties. These halides exhibit high reactivity, forming strong bonds with other elements. They possess versatile chemical behaviors, enabling their utilization in a wide range of applications.
Industrial Applications
-
-*Disinfectants and Antiseptics
Halogens, such as chlorine and iodine, are commonly used as disinfectants and antiseptics due to their ability to kill microorganisms. Chlorine is employed in water purification systems, while iodine is found in antiseptic solutions and wound dressings.
-*Bleaching Agents
Sodium hypochlorite (NaClO), a halide-based compound, is extensively used as a bleaching agent in the textile and paper industries. It effectively removes stains and whitens fabrics.
-*Flame Retardants
Certain halogenated compounds, such as brominated flame retardants, are added to plastics and other materials to enhance their fire resistance. These compounds release halogen radicals that interfere with the combustion process, suppressing flame spread.
Laboratory Applications
-
-*Analytical Chemistry
Halides are widely employed in analytical chemistry as reagents for various qualitative and quantitative analyses. Silver halides, for instance, are used in the detection of chloride ions in precipitation reactions.
-*Spectroscopy
Halides are useful in spectroscopy techniques such as ultraviolet-visible (UV-Vis) and X-ray photoelectron spectroscopy (XPS). The electronic transitions within their energy levels provide valuable information about their molecular structure and chemical bonding.
Synthesis and Characterization of Halides with 4 Energy Levels: I Am A Halide With 4 Energy Levels
The synthesis of halides with 4 energy levels involves various methods, including chemical vapor deposition (CVD), molecular beam epitaxy (MBE), and solution-based techniques. CVD utilizes the reaction of halide precursors with a metal or semiconductor substrate at high temperatures, while MBE employs a molecular beam to deposit halide materials onto a substrate under ultra-high vacuum conditions.
Solution-based methods involve the precipitation of halide compounds from a solution.
The characterization of these halides employs a range of techniques, including X-ray diffraction (XRD) for determining crystal structure, photoluminescence (PL) and absorption spectroscopy for studying optical properties, and scanning electron microscopy (SEM) and transmission electron microscopy (TEM) for imaging the morphology and microstructure.
Spectroscopic Characterization
Spectroscopic techniques provide insights into the electronic structure and optical properties of halides with 4 energy levels. PL spectroscopy measures the emission of light from the material when excited by a light source, revealing information about the bandgap and defect states.
Absorption spectroscopy determines the absorption of light by the material, providing data on the electronic transitions and energy levels.
Microscopic Characterization
Microscopic techniques offer visualization of the halides’ morphology and microstructure. SEM employs a focused electron beam to scan the surface of the material, generating images that reveal surface features, grain size, and defects. TEM utilizes a high-energy electron beam to penetrate the material, providing detailed images of the internal structure, including crystal defects and interfaces.
Halides in Chemistry
Halides are a class of inorganic compounds that contain at least one halogen atom, such as fluorine, chlorine, bromine, iodine, or astatine. They are typically ionic compounds, with the halide anion having a negative charge and the counterion having a positive charge.
Halides are widely used in various chemical industries, including the production of plastics, dyes, pharmaceuticals, and fertilizers. They also have applications in photography, water treatment, and as catalysts in chemical reactions.
Examples of Halides in Chemistry
The following table provides examples of different halides used in chemistry, along with their chemical formulas, applications, and unique properties:
| Halide | Chemical Formula | Applications | Unique Properties |
|---|---|---|---|
| Sodium chloride | NaCl | Table salt, food preservative | High solubility in water |
| Potassium chloride | KCl | Fertilizer, substitute for salt | Lowers blood pressure |
| Calcium chloride | CaCl2 | Deicing agent, drying agent | Hygroscopic (absorbs moisture from the air) |
| Magnesium chloride | MgCl2 | Deicing agent, fertilizer | Highly soluble in water |
| Sodium fluoride | NaF | Toothpaste, drinking water additive | Prevents tooth decay |
| Potassium iodide | KI | Iodized salt, photographic film | Prevents iodine deficiency |
| Silver chloride | AgCl | Photography, jewelry | Light-sensitive |
| Sodium bromide | NaBr | Sedative, photographic film | Calming effect |
| Potassium bromide | KBr | Sedative, photographic film | Calming effect |
| Sodium iodide | NaI | Radioactive tracer, medical imaging | Radioactive |
Role of Halides in Biological Systems
Halides, such as chloride (Cl –), fluoride (F –), bromide (Br –), and iodide (I –), play vital roles in various biological systems, contributing to their normal functioning and maintenance of homeostasis.
These halides participate in a range of physiological processes, including enzyme catalysis, ion transport, and neurotransmission, and their involvement is crucial for the proper functioning of organisms.
Enzyme Catalysis
Halides act as cofactors for several enzymes, facilitating their catalytic activity. For example, chloride ions are essential for the activity of α-amylase, an enzyme that breaks down starch into smaller sugar molecules. Halides stabilize the enzyme’s structure and promote substrate binding, enhancing its catalytic efficiency.
Ion Transport
Halides are involved in the active transport of ions across cell membranes, maintaining electrochemical gradients. The sodium-potassium pump, a membrane protein, utilizes a gradient of sodium and potassium ions to transport three sodium ions out of the cell and two potassium ions into the cell, establishing an electrochemical gradient across the membrane.
This gradient is essential for various cellular processes, including nerve impulse propagation and muscle contraction.
Neurotransmission
Chloride ions play a significant role in neurotransmission, regulating the excitability of neurons. Chloride channels in the neuronal membrane allow chloride ions to flow in and out of the cell, influencing the neuron’s resting membrane potential and response to stimuli.
Alterations in chloride ion homeostasis can affect neuronal excitability and contribute to neurological disorders.
Clarifying Questions
What are the key characteristics of halides with 4 energy levels?
Halides with 4 energy levels possess a unique electronic configuration that distinguishes them from other halides. This specific arrangement influences their physical and chemical properties, resulting in distinct characteristics.
How do halides with 4 energy levels compare to halides with different energy level configurations?
Halides with 4 energy levels exhibit contrasting properties compared to their counterparts with varying energy level configurations. These differences manifest in their physical and chemical attributes, offering a diverse range of applications.
What are the practical applications of halides with 4 energy levels?
Halides with 4 energy levels find applications in various fields, including optics, electronics, and catalysis. Their unique properties make them valuable components in diverse technologies and industrial processes.